October 31, 2019
Biochemistry meets mechanics: the sensitive nature of cell-cell contact formation in embryo development
IST Austria PhD student identified mechanosensitive cell-cell junctions required for cell movement in early zebrafish embryo – Study published in Cell
© Justine Renno
There is increasing evidence that the concerted actions of mechanical and biochemical signals drive key cellular processes. While the biochemical basis of these processes is quite well understood, comparably little is yet known about the mechanical forces involved. In their recent study published in Cell, PhD student Cornelia Schwayer and colleagues from the group of developmental biologist Carl-Philipp Heisenberg at the Institute of Science and Technology Austria (IST Austria) together with collaborators from the University College London, UK, have identified a novel mechanism by which biochemical and mechanical signals cooperate in cell-cell contact formation within the early zebrafish embryo—involving protein phase separation as a key feature of this process.
After the fertilization of a fish egg, the first cell divisions give rise to a mound of cells, which settle on the egg’s yolk. These cells will divide further and form different kinds of tissues. To do so, they first need to rearrange and spread out in a way that they end up covering the whole of the yolk’s surface. This cell movement is supported by contracting actomyosin structures in the yolk—similar to their well-known counterparts from muscle contraction—that drag the cells along the egg’s surface until a thin layer of embryonic cells engulfs the yolk mass. How these actomyosin filaments dock to the surface epithelial cells to exert the dragging forces, however, has remained unknown. To find out, Schwayer and colleagues analyzed a number of proteins that could potentially act as a connecting element—and identified a specific cell-cell junction protein by the name of ZO-1.
Watch the corresponding video on YouTube
Imaging of tissue spreading during early zebrafish development. Red signal displays membrane labeling outlining epithelial cells during their movement to engulf the yolk cell.
© IST Austria – Heisenberg group
Mechanical forces drive positive feedback loop
For a long time, ZO-1 has predominantly been considered as a component of tight junctions that form between cells to limit the passage of molecules and ions and to maintain proper epithelial polarity. “It came as a big surprise to us that a tight junction protein like ZO-1 can do much more than just regulating tissue sealing and polarity during homeostasis”, says Schwayer, who is in her final stage of her PhD program. “In fact, ZO-1 is sensitive to mechanical signals and thereby allows these signals to influence tight junction formation.”
Watch the corresponding video on YouTube
Phase-separated ZO-1 protein clusters flow towards the yolk-tissue boundary, where they get incorporated into tight junctions.
© IST Austria – Heisenberg group
ZO-1 responds to and scales with tension generated by the actomyosin cytoskeleton of the yolk cell to which tight junctions are connected: the higher the tension, the more ZO-1 accumulates at the forming junction. In turn, during this process of tight junction formation and maturation, ZO-1 also controls actomyosin network tension and flow within the yolk cell. “This positive feedback loop between tight junction and actomyosin network formation points at a still underestimated crosstalk between mechanical and biochemical signals in tight junction formation”, Schwayer concludes.
Phase separation as a condition for ZO-1 flow
While delving deeper into the mechanosensitive mechanism of tight junctions, Schwayer and colleagues encountered another surprise: “By using super-resolution imaging techniques, we found that ZO-1 proteins behave very much like liquid droplets during phase separation—similar to what happens when oil and vinegar de-mix in a salad dressing“, says Schwayer. Phase separation is a concept originally coming from soft matter physics. In the tight junction forming process studied here, this phenomenon helps ZO-1 respond to tension: the protein is only transported and integrated into tight junctions by the actomyosin flow within the yolk cell when it is phase-separated—or condensed into small droplets through fusion events—on the surface of the yolk.
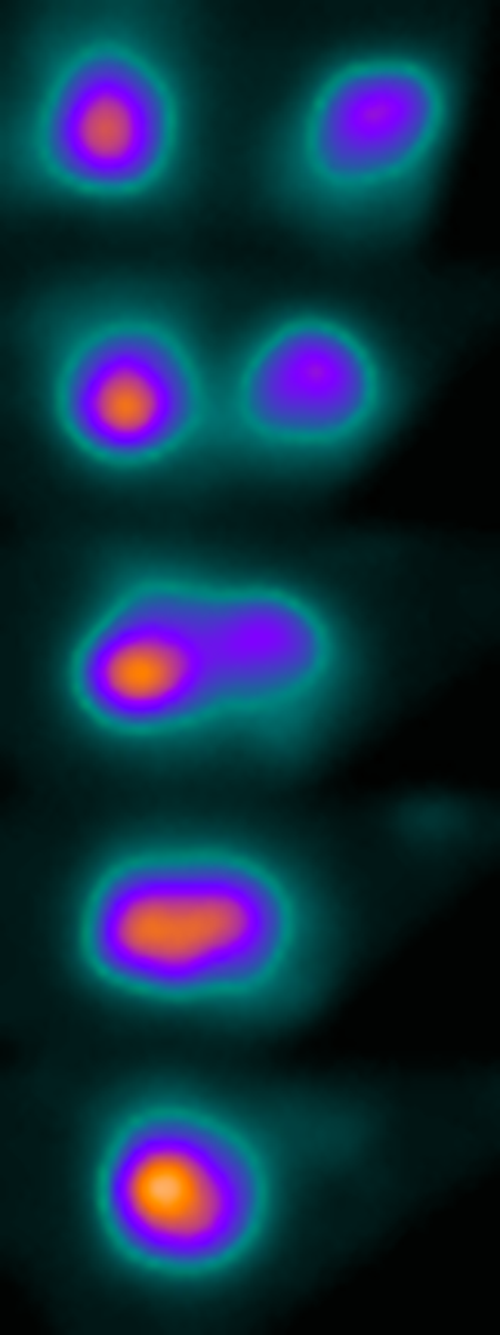
© IST Austria – Heisenberg group
Stronger together: when biology meets physics
Applying physical concepts like phase separation to biological questions is indispensable for providing truly mechanistic insight into complex biological processes, such as cell-cell contact formation. Schwayer et al.’s identification of the mechanistic basis of tight junction mechanosensitivity constitutes another example of interdisciplinary research at the interface between biology and physics—a characteristic and prolific field of curiosity-driven basic research at IST Austria.
Publication
Cornelia Schwayer, Shayan Shamipour, Kornelija Pranjic-Ferscha, Alexandra Schauer, Maria Balda, Masazumi Tada, Karl Matter & Carl-Philipp Heisenberg. 2019. Mechanosensation of Tight Junctions Depends on ZO-1 Phase Separation and Flow. Cell. DOI: 10.1016/j.cell.2019.10.006
Grant information
The IST Austria project part was supported by funding from the European Union (ERC Advanced Grant no. 742573).



