May 3, 2023
Dancing Styles of Atoms
How ISTA scientists study molecules with NMR spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy is a scientific method to unriddle the structural details of molecules and is one of the latest techniques on campus. With a recent publication by the Schanda group and the NMR symposium taking place at the Institute of Science and Technology Austria (ISTA), there is a buzz around it. But how does NMR work, why is it important for scientists, and what’s dancing got to do with it?

Among the Scientific Service Units (SSUs) at ISTA, there is a spectacular hall resembling a space station filled with huge superconducting magnets, cooling units, pumps, and compressors: the NMR Facility.
It may look like a space station, but in fact, it is a much-used facility for chemists and biochemists on campus. For them, NMR spectroscopy is a method to study molecules of all sizes: from very small ones to large biomacromolecules, such as proteins or DNA. With this tool, researchers gain insights into the physical, chemical, and biological properties of molecules.
NMR — A Radio Station for Atoms
To be clear: The word “nuclear” in NMR has nothing to do with nuclear power. It refers to the nuclei (center) of atoms—tiny building blocks that make up all materials. The magnetic property of those nuclei is responsible for nuclear magnetic resonance—a physical effect, where the nuclei of any material absorb and emit electromagnetic waves when they are exposed to large external magnetic fields. This lays the foundation of NMR spectroscopy.
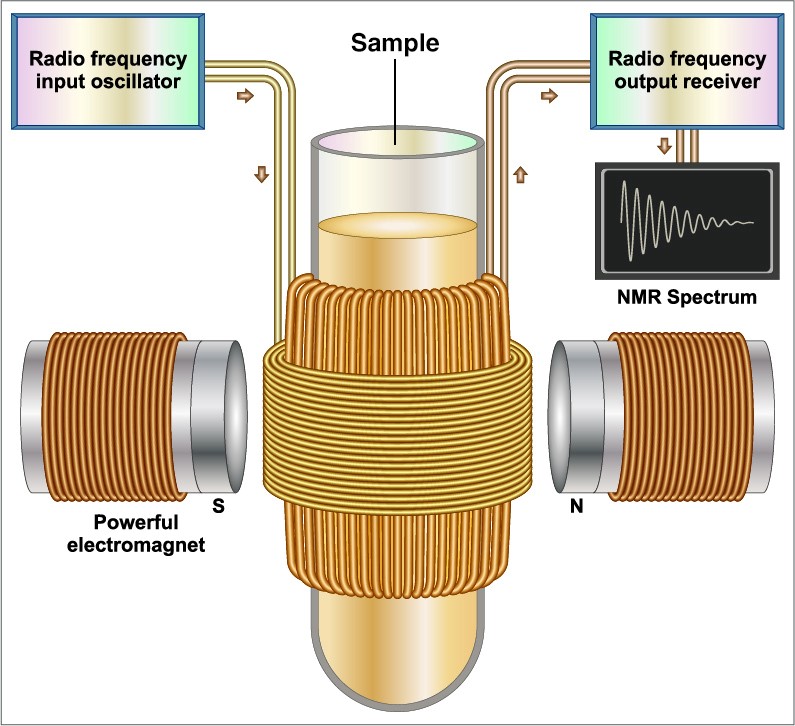
To investigate the molecules, a sample is placed into the magnet, where it gets excited with electromagnetic waves—their response is then recorded and becomes the NMR spectrum. Petra Rovó, the NMR Facility Manager, explains, “We use radio-frequency irradiation. These wavelengths are the same that the local radio stations use.” She says that the atoms in the molecules all have their favorite “tune”, which they “hear” from the NMR radio station. “Each atom has a unique ‘dancing style’ which results in a slightly different frequency of jiggling and jumping. We then record the dance of all the atoms, and with a mathematical trick, we can read out each frequency individually,” Rovó continues. The individual frequencies provide information about neighboring atoms and groups of atoms within a compound, eventually allowing the construction of a 3D representation of the molecular structure.

NMR meets Biochemistry
ISTA Professor and biochemist Paul Schanda uses NMR spectroscopy regularly. His research group is interested in how biomolecules like proteins perform their tasks. Proteins are long molecules made from chains of amino acids, and are responsible for almost everything that a biological cell does, which eventually allows life to unfold: they perform chemical reactions, convert light in our eyes to electrical signals, and many more things.
The correct execution of these processes is crucial for a human’s health—even small changes in a protein’s structure can have severe consequences. “Proteins can exist in a different architecture than what they should, for example when they form deposits in the brain of Alzheimer’s patients, known as amyloid fibrils—long filamentous structures with immense importance for neurological pathologies,” Schanda explains.
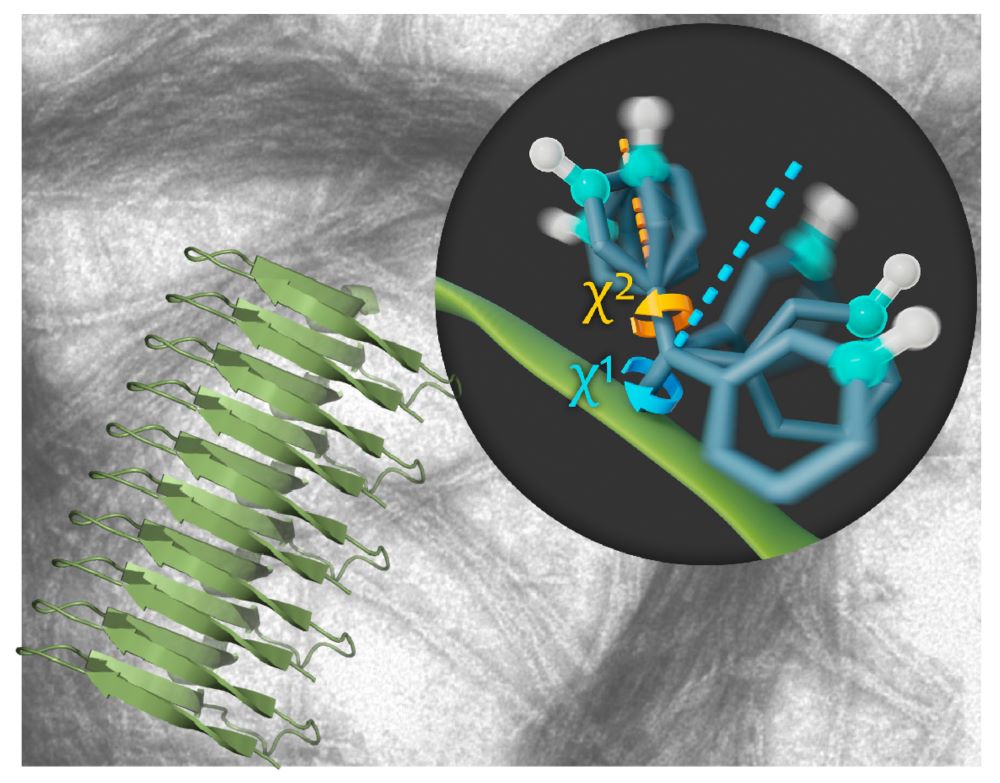
In their latest study, the group investigated how these amyloid fibrils move by using a very special high-resolution NMR spectroscopy method. “In general, molecules are not static. They are constantly in motion—for many proteins, these motions are essential for their function,” Schanda continues, “and NMR is a powerful method to study these motions. It allows us to ‘see’ the movements of individual atoms or amino-acid side chains within a 3D structure.” The results present an unprecedented look into the fibril structure and show that they are formed by a very rigid core and rapidly moving side chains on the outside.
Bringing Scientists Together
ISTA’s vibrant campus currently hosts its Nuclear Magnetic Resonance Symposium from May 2 to 3, which is followed by a two-day workshop for PhD students. “The symposium brings together some of the leaders of biological NMR spectroscopy and will be of interest to a broad range of researchers working in structural biology, biochemistry, and chemistry,” Schanda says. Invited speakers present the latest research and give young scientists and attendees creative boosts for their work. Rovó adds, “It’s an excellent opportunity to expose the NMR Facility to the world and to network. I envision new collaborations and many shared publications together with the groups who are joining the conference.”
Publication:
L. M. Becker, M. Berbon, A. Vallet, A. Grelard, E. Morvan, B. Bardiaux, R. Lichtenecker, M. Ernst, A. Loquet & P. Schanda. 2023. The Rigid Core and Flexible Surface of Amyloid Fibrils Probed by Magic-Angle-Spinning NMR Spectroscopy of Aromatic Residues.Angewandte Chemie. DOI: https://doi.org/10.1002/anie.202219314
Funding information:
The ISTA project part was supported by funding from the European Research Council (StG-2012-311318 to Paul Schanda).



